HySORB™ – The Integrated CO2 and SO2 Capture Solution Solution for Coal Based Power Industry
No Compression. No Transportation. No Storage.
Just one system that captures CO₂ + SO₂ and turns them into value.
One System. Two Pollutants. Zero Compromise.
HySORB™ is a breakthrough emissions control technology that eliminates the need for both Flue Gas Desulfurization (FGD) and standalone Carbon Capture units. Instead, it delivers a single, integrated solution that simultaneously captures sulfur dioxide (SO₂) and carbon dioxide (CO₂) in one compact process — at lower cost, with higher efficiency, and with commercially valuable byproducts.
Why SO₂ is the Hidden Barrier to Carbon Capture
One of the biggest hurdles in post-combustion carbon capture is sulfur dioxide (SO₂) in flue gas. Conventional amine-based CO₂ capture systems are extremely sensitive to SO₂ — even small amounts lead to the formation of heat-stable salts, which degrade the solvent, increase energy demand, and drive up maintenance costs. To prevent this, global carbon capture plants first rely on wet flue gas desulfurization (FGD), followed by a costly deep FGD stage to bring SO₂ levels down to as little as 1–2 ppm before CO₂ capture can even begin. This is where Indian power plants face a unique challenge. Typical coal-based flue gas in India contains 200–500 ppm SO₂, often averaging ~250 ppm, which is far higher than the tolerance limits of conventional capture systems. Without installing both FGD and deep FGD — a multi-stage setup that demands massive space and capital — CO₂ capture becomes practically impossible. HySORB™ eliminates this barrier by directly handling raw flue gas with high SO₂ levels, making simultaneous SO₂ and CO₂ capture possible in a single step — no deep FGD required.
A Third Way: The HySORB™ Integrated Solution
HySORB™ eliminates the need for both FGD and separate carbon capture units. Instead, it uses a dual-alkali slurry (sodium hydroxide + calcium oxide/hydroxide) in a single absorber vessel to directly handle raw flue gas.
Here’s how it works:
-
SO₂ Capture → Neutralized into calcium sulfite (CaSO₃) or sulfate (CaSO₄).
-
CO₂ Capture → Absorbed by NaOH, mineralized into calcium carbonate (CaCO₃). Self-Regenerating Cycle → NaOH regenerates in situ, avoiding costly solvent replacement or thermal regeneration.
-
Valuable Byproducts → High-purity precipitated calcium carbonate (PCC) is recovered, opening new revenue streams.
Why Choose HySORB™?
✅ Integrated Dual Capture – Simultaneously removes SO₂ and CO₂ in one absorber.
✅ No FGD, No Deep FGD, No Standalone Carbon Capture Unit.
✅ Retrofit-Friendly – Works with existing wet FGD towers by just changing slurry chemistry.
✅ Low Physical Footprint – Ideal for space-constrained plants.
✅ Lower Cost & Energy Demand – No reboilers, no steam, no compression load.
✅ Self-Sustaining Chemistry – In-situ NaOH regeneration reduces reagent costs.
✅ Marketable Byproducts – Generates PCC, used in paper, paints, plastics, and construction.
✅ Scalable Performance – CO₂ capture tunable from 10–90% by adjusting NaOH concentration.
Who Is It For?
HySORB™ is designed for industries that face stringent emission targets but cannot afford the cost, space, or complexity of conventional systems:
-
Coal-fired thermal power plants
-
Cement kilns
-
Steel plants
-
Chemical manufacturing
-
Other emission-intensive facilities
Environmental & Economic Impact Deep Decarbonization:
-
Cuts SO₂ to <10 ppm and captures up to 90% of CO₂.
-
Circular Value: Turns waste CO₂ into useful calcium carbonate products.
-
Cost-Effective: Eliminates multi-stage towers, reducing CapEx and OpEx.
-
Sustainable: Closed-loop chemistry minimizes waste and water usage.
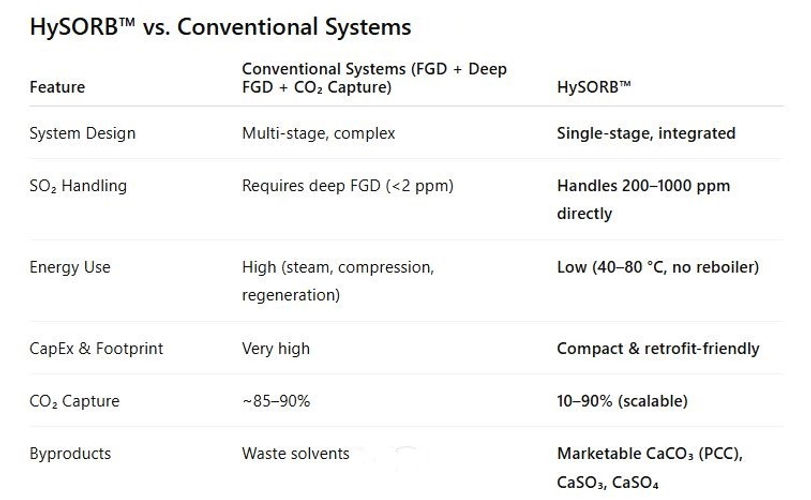
The HySORB™ Advantage
With HySORB™, industries don’t need to choose between:
-
Costly, multi-stage setups, or
-
Doing nothing and risking regulatory penalties.
Instead, HySORB™ delivers a third solution — an integrated, scalable, and profitable approach to tackling both desulfurization and decarbonization at once.
👉 HySORB™ makes industrial decarbonization practical, affordable, and sustainable.
